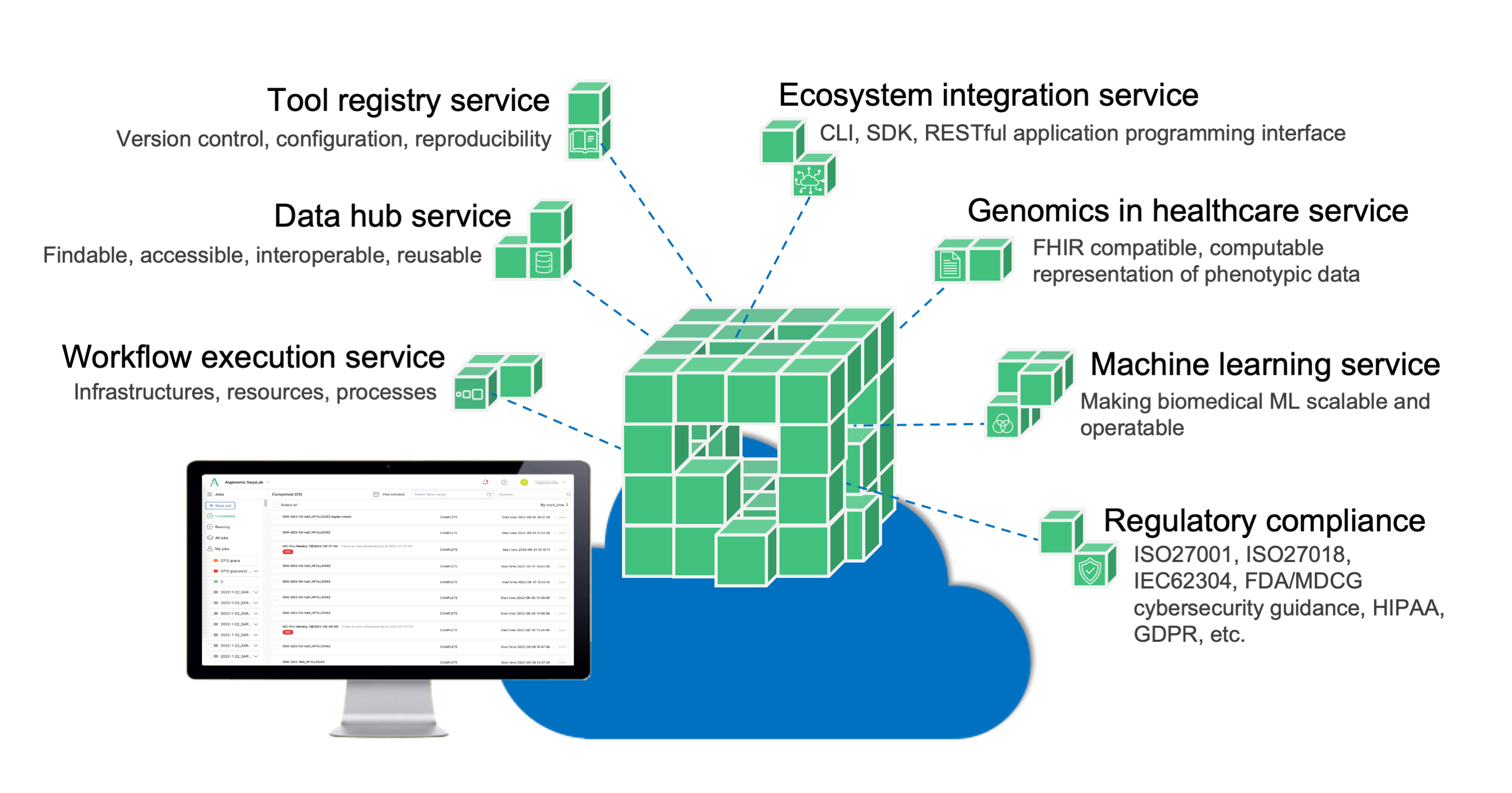
Compliance#
Atgenomix SeqsLab follows the most relevant security frameworks and regulations in the healthcare industry:
ISO/IEC 27001:2013 Information Security Management System
ISO/IEC 27018:2019 Practices for Protecting Personally Identifiable Information (PII) in Public Clouds Acting as PII Processors
IEC 62304:2006+A1:2015 Medical Device - Software Life Cycle Processes
FDA Cybersecurity Guidance
FDA 21 CFR Part 11 Audit Trail
Security measures used on SeqsLab include:
Virtual private cloud
Role-based access control
Encryption at rest and in transit
Activity audit logs
Data, code, and execution integrity
Open standards
Code inspection
Combined with Microsoft Azure’s trusted cloud, SeqsLab is compliant with the following regulations out of the box:
Health Information Trust Alliance (HITRUST)
Health Insurance Portability & Accountability Act (HIPAA)
General Data Protection Regulation (GDPR)
ISO/IEC 27018 Code of Practice for Protecting Personal Data in the Cloud
As a Gold Member in Health Level Seven International (HL7)( ), Atgenomix also supports the HL7 objective to create a suite of standard-based technologies for streamlining sophisticated data management and computational analysis of biomedical and multi-omics information.
), Atgenomix also supports the HL7 objective to create a suite of standard-based technologies for streamlining sophisticated data management and computational analysis of biomedical and multi-omics information.